Brucite is named after “A. Bruce,” an early American mineralogist name.
The Chinese name is called “水镁石” or “氢氧镁石” according to the chemical composition of the mineral.
Brucite occurs in metamorphic limestone, serpentine, and gneiss and belongs to low-temperature hydrothermal alteration minerals.
It is a plate-like crystal, mainly in the form of leaf-like, fibrous and granular aggregates. The crystal colors are white, gray, and light blue. It will be yellow or maroon If iron and manganese are doped in it.
The crystal surface has white streaks and pearl luster and is transparent. Brucite is one of the important sources of magnesium, mainly produced in the United States, France, and Britain.
- Composition: Mg (OH) 2, containing a small amount of Fe and Mn.
- Form: It belongs to the trigonal crystal system. The crystals are usually thick plate-like, and leaf-shaped. Complete cleavage with {0001} .
- Optical property: white, green, and brown on the specimen, pearl luster on the cleavage surface. Colorless in the flakes. No=1.559~1.566,Ne=1.580~1.585,Ne-No=0.019~0.021. The highest interference color is Class I purplish red, and the abnormal interference color of reddish brown tone often appears. It is parallel extinction. Negative ductility. One axis (+).
- Change: easy to change into hydromagnesite and serpentine.

What is Brucite?
The chemical formula of Brucite is Mg (OH) 2, and the hardness is 2.5. The single crystal is in the form of a thick plate, which is commonly seen as sheet aggregate, sometimes as fibrous aggregate, called Nemalite.
Theoretical composition (w%): MgO(69.12), H2O(30.88). Fe, Mn, Zn, Ni, and other impurities often exist in the same phase as the same substance.
Among them, MnO can reach 18%, FeO can reach 10%, and ZnO can reach 4%; It can form varieties of ferrobrucite (FeO ≥ 10%), manganobrucite (MnO ≥ 18%), brucite-containing zinc (ZnO ≥ 4%), brucite containing Manganese zinc (MnO 18.11%, ZnO 3.67%), brucite nickel (NiOW4%), etc.
The reliable service temperature of Brucite is 400 ℃. The thermal conductivity of Nemalite is 0.46W/(m · K), and the loose fiber is 0.131 ~ 0.213W/(m ・ K) (bulk density 0.47g/cm). The longitudinal thermal expansion of Nemalite is 16.7 × 10-7 ℃, horizontally is 8.8*10-7/℃, and the thermal expansion relation is basically linear.
Nemalite also has flame retardancy properties, resistance to an open flame, and high-temperature flame. The decomposition temperature of Nemalite is 450 ℃.
Nemalite is the best alkali-resistant natural inorganic fiber. However, it can be completely dissolved in strong acid and can be dissolved at different rates in oxalic acid, citric acid, acetic acid, mixed acid, and A1 (OH) 3 solution. In a humid or rainy climate, Nemalite is easily eroded by CO2 and HO in the atmosphere, so Brucite Products’ surface needs a waterproof protective layer.
Basic properties of Brucite
[Chemical composition] Mg (OH) 2. MgO:69.12%,H2O:30.88%。 In the composition, Fe, Mn, and Zn-like substances can replace Mg, FeO can be up to 10%, MnO can be up to 20%, and Zn can be up to 4%.
[Crystal structure] Trilateral crystal system; ah=0.3148nm,ch=0.4769nm,Z=1。 Spectral lines of main powder crystals: 2.365 (100), 4.77 (90), 1.794 (55). The brucite-type structure is one of the typical layered structures.
Mg2+of brucite(Mg [OH] 2) is distributed on the corner top of the space lattice, which is the original lattice. The two layers of 0H are the most closely packed hexagonal, and Mg2+fills all the octahedral spaces, forming the structural layer of the coordination octahedron;
The two layers of 0H that contact between the structural and structural layers are also the most closely packed in a hexagonal manner, and the octahedral space formed is not filled with cations.
There are ionic bonds in the structural layers and hydrogen bonds between the structural layers. Atomic spacing: Mg-OH=0.206, OH-OH=0.322nm.
[Crystal morphology] Compound Trigonal Trapezohedron crystal class, The crystals are often in the form of plates, scales, and leaves. The common simplex is parallel double-sided c {0001}, hexagonal column m {1120}, rhombohedron r {1011}, q {0113}, etc.
The aggregates are plate-like, flaky-like, scaly-like, and irregular granular. Sometimes it is a fibrous aggregate called Nemalite.
[Physical property] White, grayish white, reddish brown for those containing manganese or iron; Glass luster, cleavage surface pearl luster. Cleavage//{0001} Extremely complete; the cleaved sheet is flexible. The hardness is 2.5. The relative density is 2.3~2.6. It is thermoelectric. It is soluble in hydrochloric acid without bubbles.
[Microscopic characteristics] Colorless under transmission light, uniaxial crystal (+). N=1.559~1.590, N=11.580~1.600。 The refractive index increases with the increase in Fe2+content. The Nemalite is a biaxial crystal (+), 2V=20 °~42.
[Genesis ] It coexists with calcite, phlogopite, tremolite, serpentine, etc.
Type of brucite ore
Brucite ore can be divided into three main types: spherical, massive, and fibrous.
Spherical type: It is formed by hydrated from periclase, produced as a nodule, with a diameter of several millimeters to more than 20cm. The nodule is cemented by cryptocrystalline Brucite and a small amount of calcite and serpentine, with good ore quality.
Massive type: It is the product of hydrothermal alteration of magnesium-rich rocks. The ore is a crystalline granular massive aggregate coexisting with serpentine, calcite, magnesite, etc., and the content of Brucite is 30%~40%.
Fiber type: It occurs in serpentinite in vein form, with the content of Nemalite generally being 1%~9%. The mixed minerals are serpentine magnetite, and Nemalite’s purity is very high.
Where does Brucite come from?
Brucite resources in the world are often associated with magnesite, carbonate rock, and serpentinite, mainly distributed in Nevada, Arizona, North Korea, and Russia. In addition, Britain, Ireland, and Canada also have brucite resources.
China has found some brucite deposits in Fengcheng, Kuandian, and Yingkou of Liaoning, Mudanjiang of Heilongjiang, Ji’an of Jilin, Xixia of Henan, Liuhe of Jiangsu, Heimulin of southern Shaanxi, etc.
Application of Brucite
Extraction of Mg and MgO raw materials: Mg and MgO are extracted from Brucite. The content of MgO in the ore is high, and impurities are few;
Because the decomposition temperature is low, and the volatile matter produced during heating is non-toxic and harmless, so Mg, MgO, and other products can be extracted from Brucite.
Fused magnesia: It is a special pure product required by high-tech electronic products. The periclase aggregate made from Brucite by electric melting has high thermal conductivity and good electrical insulation, and the service life of the product is increased by 2-3 times.
Light magnesium oxide: the United States, Russia, Canada, Britain, and other countries use chemical methods to extract Light magnesium oxide from low-grade brucite rock.
Dead Burned Magnesite: it is mainly used to produce magnesia refractories. Magnesia carbon bricks and magnesia chrome bricks are widely used in the modern iron and steel industry. The amount of such MgO has exceeded 1/2 of its output. The Dead Burned Magnesite is made of Brucite and has the advantages of high density (>3.55g/cm), high fire resistance (>2800 ℃), high chemical properties, and high thermal shock stability.
Reinforcing material: Nemalite can be used as a substitute for chrysotile asbestos in some fields and is used in middle-grade insulation materials such as calcium silicate board with microporous and calcium silicate board.
Chemically pure magnesium reagent: metal magnesium can be extracted to prepare pure chemical reagents such as MgCl, MgSO, Mg (NO) 2, etc.
Papermaking filler: Brucite has high whiteness, good peeling property, strong adhesion, and poor water absorption.
When it is used with calcite as filler in papermaking, the process can be changed from the acid to the alkaline method, and it can reduce the pollution of pulp and water.
Flame retardant: Nemalite has a good flame retardant effect and is an ideal non-toxic, smokeless, pollution-free, and high-temperature flame retardant.
Identification of Hetian Jade and Brucite
The rough Brucite with a sense of jade quality looks very similar to Hetian jade in appearance. The outer layer even has the characteristics of Hetian Jade – white skin, which is very deceptive.
The difference:
color
The color of Brucite is generally white to light green (bluish-white), and those containing manganese or iron are yellow or reddish brown. It is very similar to the nephrite jade by the naked eye.
structure
Brucite is mainly a fibrous structure.
gloss
The fracture surface of Brucite shows a glassy luster.
transparency
Slightly transparent.
Special properties
Easily soluble in hydrochloric acid, without foaming.
How to make nanofibers or EVA composites from Brucite
The natural fiber brucite itself is in a cluster shape. After stripping, the cluster is changed into many small single fibers.
However, Brucite is a natural form of Mg (OH) 2, so it has strong alkalinity and surface electricity. In addition, because the fiber is very thin and the surface area is large, so it is easy to agglomerate.
Therefore, how to uniformly disperse brucite fibers has become the focus of research. In addition, we must maintain its high aspect ratio in the treatment process. Otherwise, it will lose its own advantages.
The alkalinity and electrical properties of brucite fiber surface make it difficult to apply it to polymer: it has poor compatibility when directly contacting with polymer.
After many tests, it was found that silane had a good modification effect on the fiber, which can improve its compatibility with EVA and improve its composite tensile strength and elongation at break.
EVA has good flexibility, high elasticity, tear and puncture resistance, low density, and good electrical and chemical stability, and it is widely used in cable sheaths, seals, medical tubes, insulating films, and other materials. The cable protective layer made of polymer materials often causes fire, so its flame retardancy is very important.
Main raw materials
- EVA,18-3;
- Fiber brucite;
- Dispersant: sulfonated organic ester salt with eighteen carbon chains;
- Surface modifier, silane No. 1 and silane No. 2.
Preparation of Brucite Fiber
Raw Brucite → fine crushing → chemical agent action → mechanical stripping →Dispersant treatment → separation and purification → screening → ultra-fine classification → nanofibers.
Surface Modification of Brucite Fiber
The obtained brucite nanofibers were washed six times in deionized water to remove the Dispersant attached to the fiber surface;
Then add silane surface modifier according to 1%~4% of the dry sample weight of the fiber in the suspension of brucite fiber, and then stir at high speed for 15 min in a high-speed mixer at the speed of 5 gears.
Pour it into the beaker and let it stand. The solid material of the suspension is gradually separated from the water, and the fiber floats on the water’s surface in a fluffy form.
Please take out the solid material, place it in the oven and bake it at 100 ℃ for 6h; completely remove the moisture, and make the coupling agent and the surface of brucite fiber fully bonded.
After the sample is cooled, the loose cake is obtained, which is dispersed on the agitator and sieved through a 200 mesh sieve so that there is loose agglomeration in the subsequent experiment. Finally, nanofiber powder is obtained for future use.
Preparation of Brucite Nanofiber/EVA Composites
Weigh the modified brucite nanofiber and EVA in proportion, heat the front roll of 5K-160B open-type plastic mill to 120~130 ℃, soften the EVA in the open mill, and then add brucite nanofiber powder.
Plaster on the open mill for 15-20 min until the color and fluidity of the composite are uniform. In this way, brucite nanofibers/EVA composites are obtained.
Then, the standard sample can be made after being pressed by the flat vulcanizing machine for mechanical property test.
Dispersion analysis in processing
1. Dispersion of brucite nanofibers
In the preparation and application of nanomaterials, dispersion is the first key problem to be solved. Similarly, in the process of preparing brucite nanofibers, the prepared nanofibers must be kept in a uniform dispersion state.
Otherwise, the coating of the modifier will be incomplete in the later modification process, and it will be filled into the polymer to form loose aggregates, reducing the mechanical properties of the composite.
The surface of Brucite is a layer of highly polar base, and because the fiber fineness reaches the nanometer level and the aspect ratio is large, it is easy to agglomerate in water to form flocs.
Dispersants commonly used for micron particles can not achieve the desired effect. After a large number of experiments, it was found that a sulfonated organic ester salt with a carbon chain of eighteen had a good dispersion effect on this nanofiber.
The brucite fibers without dispersant treatment are seriously agglomerated, while the brucite fibers treated with Dispersant have good dispersion.
Obviously, this Dispersant has played a good role in dispersion and solved the problem of agglomeration of nanofibers.
2. Surface modification of brucite nanofibers and their dispersion in EVA
How to dry brucite nanofibers uniformly dispersed in water, fill them into polymer materials, and maintain good dispersion is a major problem in the application of nanopowder or fiber filler.
Generally, spray drying is used, but its disadvantage is obvious – energy consumption is very high. After repeated exploration, the above-mentioned wet modification technology was used to solve this problem.
Due to avoiding the process of filtering and caking during the processing, the prepared brucite nanofibers show a good loose dispersion state.
The surface modification of brucite nanofibers and their dispersion in organic polymers determine the mechanical properties of this nanocomposite.
According to the surface modification method, a better modification effect and dispersion state were obtained. When the addition amount was 5% and 20%, the fibers were well dispersed in EVA without agglomeration.
In addition, the fiber still maintains a high aspect ratio (about 20:1) without breaking during processing.
In addition, when the fiber addition amount is 5%, the fibers are arranged in a disorderly manner, with little directivity. When the fiber content is 20%, the fiber arrangement is obviously directional, and the arrangement is quite neat.
It is estimated that due to the increase of the additional amount and the fiber diameter at the nanometer level, the number of fibers in the unit volume is large, resulting in a relatively small activity space for each fiber in the polymer fluid.
Because the fiber length is much larger than the diameter, the fiber is restricted by adjacent fibers when rotating, resulting in the directional arrangement of fibers. This can play a role in some composites requiring high strength in one dimension direction.
The transition between the fiber edge and EVA is very smooth, without cracks and clear boundaries, indicating that the fiber and EVA are firmly bonded by the coupling agent without incompatibility.
Due to the rigidity of the fiber, the fiber protrudes from the EVA, and the angle of the joint part of the fiber and EVA is a concave arc; that is, the contact angle is a small acute angle close to the full immersion state.
This strongly demonstrates the affinity and tight bonding between surface-modified brucite nanofibers and EVs.
Mechanical Properties and Flame Retardancy of Brucite Nanofiber/EVA Composites
The above figure is a comparison chart of tensile strength measured by adding 5% of brucite nanofibers into EVA after being modified with different modifiers at different dosages.
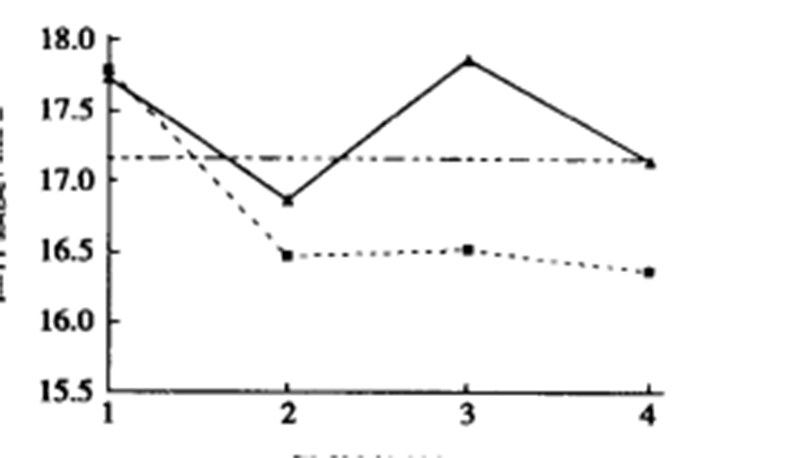
From the figure-1, we can know that when the amount of coupling agent is 1%, the modification effect is good; The effect of silane No.2 at 3% was obviously better than that of silane No.1 and was better than that at 1%;
In addition, with the increase of the amount of coupling agent, the strength of the composite has a downward trend, which may be caused by the excessive amount of coupling agent and the lubricating effect of residual organic small molecules in the polymer.
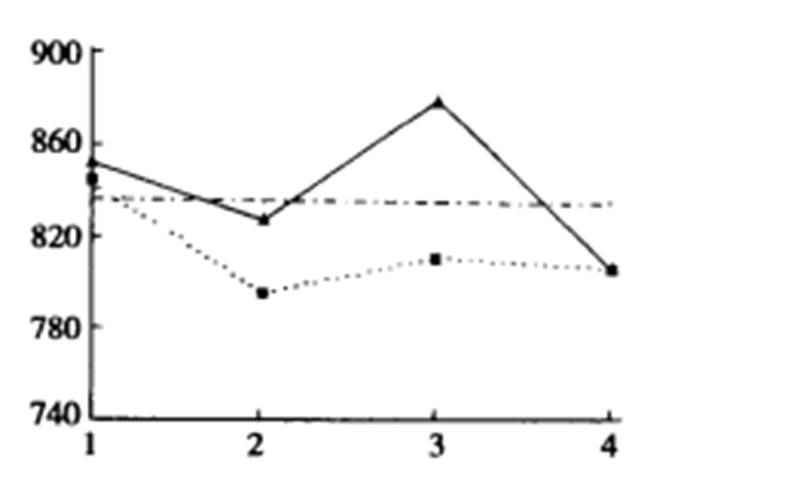
From figure-2, we can know that when the dosage is 3%, silane No.2 has the best modification effect on brucite fiber, significantly improving its elongation at break; in contrast, the modification of Silane No. 1 is less effective.
Based on the results in Fig. 1 and Fig.2, we can know that silane No.2 is suitable for the modification of brucite nanofibers, which significantly improves the strength and tensile properties of the composite.
At the same time, it also shows that brucite nanofibers prepared by this method can improve the mechanical properties of EVA to a certain extent and provide a feasible solution to the problem of mechanical properties decline in the application of brucite flame retardant in the polymer field.
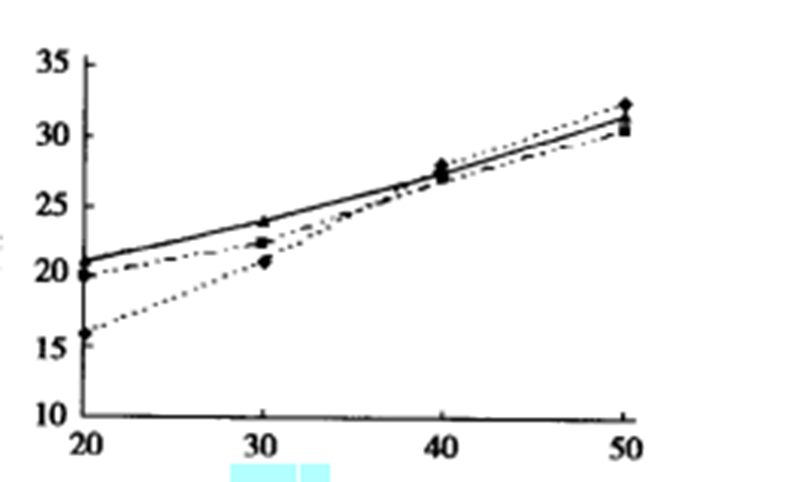
From Figure 3, we can know that the oxygen index of EVA composite made of brucite nanofibers treated with silane No. 2 is always higher than that of EVA composite made of untreated brucite nanofibers. It may have a certain relationship with the degree of dispersion of fibers in the composite material and the degree of bonding between the inorganic and organic phases.
In the case of low filling amounts (such as 20% and 30%), the oxygen index of the composite filled with nanofibers is higher than that of the composite filled with micron-sized magnesium hydroxide particles;
In the case of high filling amount (such as 50%), the oxygen index of the composite filled with nanofibers is slightly lower than that of the composite filled with micron magnesium hydroxide particles.
This may be due to the purity of micron-grade Brucite being higher than that of the prepared brucite nanofibers.
The difference is hidden by the advantages of the high dispersion, high homogeneity, and high interface area of the inorganic phase in the composite filled with nanofibers when the filling amount is low. However, this difference is revealed only when the filling amount is high.
On the whole, the flame-retardant performance of EVA composites filled with brucite nanofibers is better than that of EVA composites filled with micron-sized magnesium hydroxide particles, especially in the case of low filling amounts.
Effect
- Under a certain mechanical force, the iodized organic ester salt with a carbon chain of 18 can be used as a dispersant to process the natural fibrous Brucite to the nanometer level and uniformly disperse it in water.
- Non-agglomerated brucite nanofiber powder can be prepared by wet surface modification, and the nanofibers can be uniformly dispersed into EVA by conventional industrial equipment.
- The modified brucite nanofibers have good compatibility with EVA and are firmly bonded.
- After the surface treatment of silane coupling agent Silane Np.2, brucite nanofibers can be used in EVA to improve its mechanical properties.
- In the case of low filling amount, the oxygen index of EVA filled with treated brucite nanofibers is higher than that of EVA filled with Brucite.
Conclusion:
Brucite is a typical low-temperature hydrothermal altered mineral in serpentinite stone or dolomite. The fibrous aggregate is called Nemalite.
Nemalite has a high aspect ratio (up to 100 at most), and the diameter of a single fiber is about 4-50 nm. As Brucite is a new low-smoke halogen-free flame retardant material developed rapidly in recent years, it is mainly used in the polymer field as a green environment-friendly flame retardant, replacing traditional flame retardants that are prone to environmental pollution, such as phosphorus and halogen;
At the same time, because its decomposition temperature is about 140 ℃ higher than that of aluminum hydroxide-another inorganic flame retardant, It has wider and more promising than aluminum hydroxide.
